Water

Water in three states: liquid, solid (ice), and (invisible) water vapor in the air. Clouds are the accumulations of the droplets, condensed from vapor-saturated air.
Water covers 70.9% of the Earth’s surface, and is vital for all known forms of life. On Earth, it is found mostly in oceans and other large water bodies, with 1.6% of water below ground in aquifers and 0.001% in the air as vapor, clouds (formed of solid and liquid water particles suspended in air), and precipitation.] Oceans hold 97% of surface water, glaciers and polar ice caps 2.4%, and other land surface water such as rivers, lakes and ponds 0.6%. A very small amount of the Earth’s water is contained within biological bodies and manufactured products.
Water on Earth moves continually through a cycle of evaporation or transpirationevapotranspiration), precipitation, and runoff, usually reaching the sea. Over land, evaporation and transpiration contribute to the precipitation over land.
Clean drinking water is essential to human and other lifeforms. Access to safe drinking water has improved steadily and substantially over the last decades in almost every part of the world. There is a clear correlation between access to safe water and GDP per capita. However, some observers have estimated that by 2025 more than half of the world population will be facing water-based vulnerability. A recent report (November 2009) suggests that by 2030, in some developing regions of the world, water demand will exceed supply by 50%. Water plays an important role in the world economy, as it functions as a solvent for a wide variety of chemical substances and facilitates industrial cooling and transportation. Approximately 70% of freshwater is consumed by agriculture.
Chemical and physical properties

Model of hydrogen bonds between molecules of water

Impact from a water drop causes an upward “rebound” jet surrounded by circular capillary waves.

Snowflakes by Wilson Bentley, 1902

Dew drops adhering to a spider web
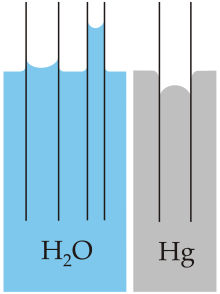
Capillary action of water compared to mercury
Water appears in nature in all three common states of matter and may take many different forms on Earth: water vapor and clouds in the sky; seawater and icebergs in the polar oceans; glaciers and rivers in the mountains; and the liquid in aquifers in the ground.
The major chemical and physical properties of water are:
- Water is a tasteless, odorless liquid at standard temperature and pressure. The color of water and ice is, intrinsically, a very slight blue hue, although water appears colorless in small quantities. Ice also appears colorless, and water vapor is essentially invisible as a gas.
- Water is transparent, and thus aquatic plants can live within the water because sunlight can reach them. Only strong UV light is slightly absorbed.
- Since the water molecule is not linear and the oxygen atom has a higher electronegativity than hydrogen atoms, it carries a slight negative charge, whereas the hydrogen atoms are slightly positive. As a result, water is a polar molecule with an electrical dipole moment. The net interactions between the dipoles on each molecule cause an effective skin effect at the interface of water with other substances, or air at the surface, the latter given rise to water’s high surface tension. This dipolar nature contributes to water molecules’ tendency to form hydrogen bonds which cause water’s many special properties.] The polar nature also favors adhesion to other materials.
- Each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; chemists call this shared electron pair a covalent chemical bond. In H2O, only two of the six outer-shell electrons of oxygen are used for this purpose, leaving four electrons which are organized into two non-bonding pairs. The four electron pairs surrounding the oxygen tend to arrange themselves as far from each other as possible in order to minimize repulsions between these clouds of negative charge. This would ordinarily result in a tetrahedral geometry in which the angle between electron pairs (and therefore the H-O-H bond angle) is 109.5°. However, because the two non-bonding pairs remain closer to the oxygen atom, these exert a stronger repulsion against the two covalent bonding pairs, effectively pushing the two hydrogen atoms closer together. The result is a distorted tetrahedral arrangement in which the H-O-H angle is 104.5°.
- A result of interplay of these properties, Capillary action refers to the tendency of water to move up a narrow tube against the force of gravity. This property is relied upon by all vascular plants, such as trees.
- Water is a good solvent and is often referred to as the universal solvent. Substances that dissolve in water, e.g., salts, sugars, acids, alkalis, and some gases – especially oxygen, carbon dioxide (carbonation) are known as hydrophilic (water-loving) substances, while those that do not mix well with water (e.g., fats and oils), are known as hydrophobic (water-fearing) substances.
- All the major components in cells (proteins, DNA and polysaccharides) are also dissolved in water.
- Pure water has a low electrical conductivity, but this increases significantly with the dissolution of a small amount of ionic material such as sodium chloride.
- The boiling point of water (and all other liquids) is dependent on the barometric pressure. For example, on the top of Mt. Everest water boils at 68 °C (154 °F), compared to 100 °C (212 °F) at sea level. Conversely, water deep in the ocean near geothermal vents can reach temperatures of hundreds of degrees and remain liquid.
- Water has the second highest molar specific heat capacity of any known substance, after ammonia, as well as a high heat of vaporization (40.65 kJ·mol−1), both of which are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow water to moderate Earth’s climate by buffering large fluctuations in temperature.
- The maximum density of water occurs at 3.98 °C (39.16 °F). It has the anomalous property of becoming less dense, not more, when it is cooled down to its solid form, ice. It expands to occupy 9% greater volume in this solid state, which accounts for the fact of ice floating on liquid water.

ADR label for transporting goods dangerously reactive with water
- Water is miscible with many liquids, such as ethanol, in all proportions, forming a single homogeneous liquid. On the other hand, water and most oils are immiscible usually forming layers according to increasing density from the top. As a gas, water vapor is completely miscible with air.
- Water forms an azeotrope with many other solvents.
- Water can be split by electrolysis into hydrogen and oxygen.
- As an oxide of hydrogen, water is formed when hydrogen or hydrogen-containing compounds burn or react with oxygen or oxygen-containing compounds. Water is not a fuel, it is an end-product of the combustion of hydrogen. The energy required to split water into hydrogen and oxygen by electrolysis or any other means is greater than the energy released when the hydrogen and oxygen recombine.
- Elements which are more electropositive than hydrogen such as lithium, sodium, calcium, potassium and caesium displace hydrogen from water, forming hydroxides. Being a flammable gas, the hydrogen given off is dangerous and the reaction of water with the more electropositive of these elements may be violently explosive.
Taste and odor
Water can dissolve many different substances, giving it varying tastes and odors. Humans and other animals have developed senses which (more or less) enable them to evaluate the potability of water by avoiding water that is too salty or putrid. The taste advertised in spring water or mineral water derives from the minerals dissolved in it: Pure H2O is tasteless and odorless. The advertised purity of spring and mineral water refers to absence of toxins, pollutants and microbes.Distribution of water in nature
Water in the universe
Much of the universe’s water may be produced as a byproduct of star formation. When stars are born, their birth is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the shock waves that are created compress and heat the gas. The water observed is quickly produced in this warm dense gas.Water has been detected in interstellar clouds within our galaxy, the Milky Way. Water probably exists in abundance in other galaxies, too, because its components, hydrogen and oxygen, are among the most abundant elements in the universe. Interstellar clouds eventually condense into solar nebulae and solar systems such as ours.
Water vapor is present in
- Atmosphere of Mercury: 3.4%, and large amounts of water in Mercury’s exosphere
- Atmosphere of Venus: 0.002%
- Earth’s atmosphere: ~0.40% over full atmosphere, typically 1–4% at surface
- Atmosphere of Mars: 0.03%
- Atmosphere of Jupiter: 0.0004%
- Atmosphere of Saturn – in ices only
- Enceladus (moon of Saturn): 91%
- exoplanets known as HD 189733 b and HD 209458 b.
- Earth – 71% of surface
- Moon – small amounts of water have been found (in 2008) in the inside of volcanic pearls brought from Moon to Earth by the Apollo 15 crew in 1971.[19] NASA reported the detection of water molecules by NASA’s Moon Mineralogy Mapper aboard the Indian Space Research Organization’s Chandrayaan-1 spacecraft in September 2009.
Water ice is present on
- Earth – mainly as ice sheets
- polar ice caps on Mars
- Moon
- Titan
- Europa
- Saturn’s rings
- Enceladus
- Pluto and Charon
- Comets and comet source populations (Kuiper belt and Oort cloud objects).
Water and habitable zone

The Solar System along center row range of possible habitable zones of varying star sizes.
Earth’s gravity allows it to hold an atmosphere. Water vapor and carbon dioxide in the atmosphere provide a temperature buffer (greenhouse effect) which helps maintain a relatively steady surface temperature. If Earth were smaller, a thinner atmosphere would allow temperature extremes, thus preventing the accumulation of water except in polar ice caps (as on Mars).
The surface temperature of Earth has been relatively constant through geologic time despite varying levels of incoming solar radiation (insolation), indicating that a dynamic process governs Earth’s temperature via a combination of greenhouse gases and surface or atmospheric albedo. This proposal is known as the Gaia hypothesis.
The state of water on a planet depends on ambient pressure, which is determined by the planet’s gravity. If a planet is sufficiently massive, the water on it may be solid even at high temperatures, because of the high pressure caused by gravity, as it was observed on exoplanets Gliese 436 b and GJ 1214 b.
Water on Earth
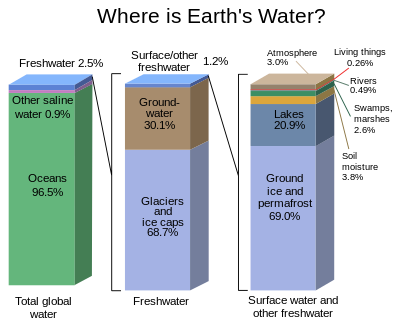
A graphical distribution of the locations of water on Earth.

Water covers 71% of the Earth’s surface; the oceans contain 97.2% of the Earth’s water. The Antarctic ice sheet, which contains 61% of all fresh water on Earth, is visible at the bottom. Condensed atmospheric water can be seen as clouds, contributing to the Earth’s albedo.
The collective mass of water found on, under, and over the surface of a planet is called the hydrosphere. Earth’s approximate water volume (the total water supply of the world) is 1,360,000,000 km3 (326,000,000 mi3).
Groundwater and fresh water are useful or potentially useful to humans as water resources.
Liquid water is found in bodies of water, such as an ocean, sea, lake, river, stream, canal, pond, or puddle. The majority of water on Earth is sea water. Water is also present in the atmosphere in solid, liquid, and vapor states. It also exists as groundwater in aquifers.
Water is important in many geological processes. Groundwater is present in most rocks, and the pressure of this groundwater affects patterns of faulting. Water in the mantle is responsible for the melt that produces volcanoes at subduction zones. On the surface of the Earth, water is important in both chemical and physical weathering processes. Water and, to a lesser but still significant extent, ice, are also responsible for a large amount of sediment transport that occurs on the surface of the earth. Deposition of transported sediment forms many types of sedimentary rocks, which make up the geologic record of Earth history.
Water cycle

Water cycle
Water moves perpetually through each of these regions in the water cycle consisting of following transfer processes:
- evaporation from oceans and other water bodies into the air and transpiration from land plants and animals into air.
- precipitation, from water vapor condensing from the air and falling to earth or ocean.
- runoff from the land usually reaching the sea.
Water runoff often collects over watersheds flowing into rivers. A mathematical model used to simulate river or stream flow and calculate water quality parameters is hydrological transport model. Some of water is diverted to irrigation for agriculture. Rivers and seas offer opportunity for travel and commerce. Through erosion, runoff shapes the environment creating river valleys and deltas which provide rich soil and level ground for the establishment of population centers. A flood occurs when an area of land, usually low-lying, is covered with water. It is when a river overflows its banks or flood from the sea. A drought is an extended period of months or years when a region notes a deficiency in its water supply. This occurs when a region receives consistently below average precipitation.
Fresh water storage


High tide (left) and low tide (right)
Sea water
Sea water contains about 3.5% salt on average, plus smaller amounts of other substances. The physical properties of sea water differ from fresh water in some important respects. It freezes at a lower temperature (about –1.9 °C) and its density increases with decreasing temperature to the freezing point, instead of reaching maximum density at a temperature above freezing. The salinity of water in major seas varies from about 0.7% in the Baltic Sea to 4.0% in the Red Sea.Tides
Tides are the cyclic rising and falling of Earth’s ocean surface caused by the tidal forces of the Moon and the Sun acting on the oceans. Tides cause changes in the depth of the marine and estuarine water bodies and produce oscillating currents known as tidal streams. The changing tide produced at a given location is the result of the changing positions of the Moon and Sun relative to the Earth coupled with the effects of Earth rotation and the local bathymetry. The strip of seashore that is submerged at high tide and exposed at low tide, the intertidal zone, is an important ecological product of ocean tides.Effects on life

An oasis is an isolated water source with vegetation in desert

Overview of photosynthesis and respiration. Water (at right), together with carbon dioxide (CO2), form oxygen and organic compounds (at left), which can be respired to water and (CO2).
Water is fundamental to photosynthesis and respiration. Photosynthetic cells use the sun’s energy to split off water’s hydrogen from oxygen. Hydrogen is combined with CO2 (absorbed from air or water) to form glucose and release oxygen. All living cells use such fuels and oxidize the hydrogen and carbon to capture the sun’s energy and reform water and CO2 in the process (cellular respiration).
Water is also central to acid-base neutrality and enzyme function. An acid, a hydrogen ion (H+, that is, a proton) donor, can be neutralized by a base, a proton acceptor such as hydroxide ion (OH−) to form water. Water is considered to be neutral, with a pH (the negative log of the hydrogen ion concentration) of 7. Acids have pH values less than 7 while bases have values greater than 7.

Some of the biodiversity of a coral reef
Aquatic life forms

Some marine diatoms – a key phytoplankton group
Aquatic vertebrates must obtain oxygen to survive, and they do so in various ways. Fish have gills instead of lungs, although some species of fish, such as the lungfish, have both. Marine mammals, such as dolphins, whales, otters, and seals need to surface periodically to breathe air. Some amphibians are able to absorb oxygen through their skin. Invertebrates exhibit a wide range of modifications to survive in poorly oxygenated waters including breathing tubes (Diptera and some molluscs)and gills (Carcinus). However as invertebrate life evolved in an aquatic habitat most have little or new specialisation for respiration in water.
Effects on human civilization

Water fountain
Health and pollution

Environmental Science Program, Iowa State University student sampling water.
Water that is not fit for drinking but is not harmful for humans when used for swimming or bathing is called by various names other than potable or drinking water, and is sometimes called safe water, or “safe for bathing”. Chlorine is a skin and mucous membrane irritant that is used to make water safe for bathing or drinking. Its use is highly technical and is usually monitored by government regulations (typically 1 part per million (ppm) for drinking water, and 1–2 ppm of chlorine not yet reacted with impurities for bathing water). Water for bathing may be maintained in satisfactory microbiological condition using chemical disinfectants such as chlorine or ozone or by the use of Ultra violet light.
In the USA, non-potable forms of wastewater generated by humans may be reffered to as greywater, which is treatable and thus easily able to be made potable again, and blackwater, which generally contains sewage and other forms of waste which require further treatment in order to be made reusable. Greywater composes 50-80% of residential wastewater generated by a household’s sanitation equipment (sinks, showers and kitchen runoff, but not toilets, which generate blackwater.) These terms may have different meanings in other countries and cultures.
This natural resource is becoming scarcer in certain places, and its availability is a major social and economic concern. Currently, about a billion people around the world routinely drink unhealthy water. Most countries accepted the goal of halving by 2015 the number of people worldwide who do not have access to safe water and sanitation during the 2003 G8 Evian summit.[26] Even if this difficult goal is met, it will still leave more than an estimated half a billion people without access to safe drinking water and over a billion without access to adequate sanitation. Poor water quality and bad sanitation are deadly; some five million deaths a year are caused by polluted drinking water. The World Health Organization estimates that safe water could prevent 1.4 million child deaths from diarrhea each year. Water, however, is not a finite resource, but rather re-circulated as potable water in precipitation in quantities many degrees of magnitude higher than human consumption. Therefore, it is the relatively small quantity of water in reserve in the earth (about 1% of our drinking water supply, which is replenished in aquifers around every 1 to 10 years), that is a non-renewable resource, and it is, rather, the distribution of potable and irrigation water which is scarce, rather than the actual amount of it that exists on the earth. Water-poor countries use importation of goods as the primary method of importing water (to leave enough for local human consumption), since the manufacturing process uses around 10 to 100 times products’ masses in water.
In the developing world, 90% of all wastewater still goes untreated into local rivers and streams. Some 50 countries, with roughly a third of the world’s population, also suffer from medium or high water stress, and 17 of these extract more water annually than is recharged through their natural water cycles. The strain not only affects surface freshwater bodies like rivers and lakes, but it also degrades groundwater resources.
Human uses
Agriculture

Irrigation of field crops
Water as a scientific standard
On 7 April 1795, the gram was defined in France to be equal to “the absolute weight of a volume of pure water equal to a cube of one hundredth of a meter, and to the temperature of the melting ice.”[32] For practical purposes though, a metallic reference standard was required, one thousand times more massive, the kilogram. Work was therefore commissioned to determine precisely the mass of one liter of water. In spite of the fact that the decreed definition of the gram specified water at 0 °C—a highly reproducible temperature—the scientists chose to redefine the standard and to perform their measurements at the temperature of highest water density, which was measured at the time as 4 °C (39 °F).The Kelvin temperature scale of the SI system is based on the triple point of water, defined as exactly 273.16 K or 0.01 °C. The scale is a more accurate development of the Celsius temperature scale, which was originally defined according the boiling point (set to 100 °C) and melting point (set to 0 °C) of water.
Natural water consists mainly of the isotopes hydrogen-1 and oxygen-16, but there is also small quantity of heavier isotopes such as hydrogen-2 (deuterium). The amount of deuterium oxides or heavy water is very small, but it still affects the properties of water. Water from rivers and lakes tends to contain less deuterium than seawater. Therefore, standard water is defined in the Vienna Standard Mean Ocean Water specification.
For drinking

A young girl drinking bottled water

Water quality: fraction of population using improved water sources by country

Hazard symbol for No drinking water
Humans require water that does not contain too many impurities. Common impurities include metal salts and oxides (including copper, iron, calcium and lead) and/or harmful bacteria, such as Vibrio. Some solutes are acceptable and even desirable for taste enhancement and to provide needed electrolytes.
The single largest (by volume) freshwater resource suitable for drinking is Lake Baikal in Siberia.
Washing
The propensity of water to form solutions and emulsions is useful in various washing processes. Many industrial processes rely on reactions using chemicals dissolved in water, suspension of solids in water slurries or using water to dissolve and extract substances. Washing is also an important component of several aspects of personal body hygiene.Chemical uses
Water is widely used in chemical reactions as a solvent or reactant and less commonly as a solute or catalyst. In inorganic reactions, water is a common solvent, dissolving many ionic compounds. In organic reactions, it is not usually used as a reaction solvent, because it does not dissolve the reactants well and is amphoteric (acidic and basic) and nucleophilic. Nevertheless, these properties are sometimes desirable. Also, acceleration of Diels-Alder reactions by water has been observed. Supercritical water has recently been a topic of research. Oxygen-saturated supercritical water combusts organic pollutants efficiently.As a heat transfer fluid

Ice used for cooling.
In the nuclear power industry, water can also be used as a neutron moderator. In most nuclear reactors, water is both a coolant and a moderator. This provides something of a passive safety measure, as removing the water from the reactor also slows the nuclear reaction down – however other methods are favored for stopping a reaction and it is preferred to keep the nuclear core covered with water so as to ensure adequate cooling.
Extinguishing fires

Water is used for fighting wildfires.
Use of water in fire fighting should also take into account the hazards of a steam explosion, which may occur when water is used on very hot fires in confined spaces, and of a hydrogen explosion, when substances which react with water, such as certain metals or hot graphite, decompose the water, producing hydrogen gas.
The power of such explosions was seen in the Chernobyl disaster, although the water involved did not come from fire-fighting at that time but the reactor’s own water cooling system. A steam explosion occurred when the extreme over-heating of the core caused water to flash into steam. A hydrogen explosion may have occurred as a result of reaction between steam and hot zirconium.
Recreation
Humans use water for many recreational purposes, as well as for exercising and for sports. Some of these include swimming, waterskiing, boating, surfing and diving. In addition, some sports, like ice hockey and ice skating, are played on ice. Lakesides, beaches and waterparks are popular places for people to go to relax and enjoy recreation. Many find the sound and appearance of flowing water to be calming, and fountains and other water features are popular decorations. Some keep fish and other life in aquariums or ponds for show, fun, and companionship. Humans also use water for snow sports i.e. skiing, sledding, snowmobiling or snowboarding, which requires the water to be frozen. People may also use water for play fighting such as with snowballs, water guns or water balloons.Water industry

A water-carrier in India, 1882. In many places where running water was not available, water had to be transported by people.

A manual water pump in China

Water purification facility
Drinking water is often collected at springs, extracted from artificial borings (wells) in the ground, or pumped from lakes and rivers. Building more wells in adequate places is thus a possible way to produce more water, assuming the aquifers can supply an adequate flow. Other water sources include rainwater collection. Water may require purification for human consumption. This may involve removal of undissolved substances, dissolved substances and harmful microbes. Popular methods are filtering with sand which only removes undissolved material, while chlorination and boiling kill harmful microbes. Distillation does all three functions. More advanced techniques exist, such as reverse osmosis. Desalination of abundant seawater is a more expensive solution used in coastal arid climates.
The distribution of drinking water is done through municipal water systems, tanker delivery or as bottled water. Governments in many countries have programs to distribute water to the needy at no charge. Others[wh argue that the market mechanism and free enterprise are best to manage this rare resource and to finance the boring of wells or the construction of dams and reservoirs.
Reducing usage by using drinking (potable) water only for human consumption is another option. In some cities such as Hong Kong, sea water is extensively used for flushing toilets citywide in order to conserve fresh water resources.
Polluting water may be the biggest single misuse of water; to the extent that a pollutant limits other uses of the water, it becomes a waste of the resource, regardless of benefits to the polluter. Like other types of pollution, this does not enter standard accounting of market costs, being conceived as externalities for which the market cannot account. Thus other people pay the price of water pollution, while the private firms’ profits are not redistributed to the local population victim of this pollution. Pharmaceuticals consumed by humans often end up in the waterways and can have detrimental effects on aquatic life if they bioaccumulate and if they are not biodegradable.
Wastewater facilities are storm sewers and wastewater treatment plants. Another way to remove pollution from surface runoff water is bioswale.
Industrial applications
Water is used in power generation. Hydroelectricity is electricity obtained from hydropower. Hydroelectric power comes from water driving a water turbine connected to a generator. Hydroelectricity is a low-cost, non-polluting, renewable energy source. The energy is supplied by the sun. Heat from the sun evaporates water, which condenses as rain in higher altitudes, from where it flows down.Pressurized water is used in water blasting and water jet cutters. Also, very high pressure water guns are used for precise cutting. It works very well, is relatively safe, and is not harmful to the environment. It is also used in the cooling of machinery to prevent over-heating, or prevent saw blades from over-heating.
Water is also used in many industrial processes and machines, such as the steam turbine and heat exchanger, in addition to its use as a chemical solvent. Discharge of untreated water from industrial uses is pollution. Pollution includes discharged solutes (chemical pollution) and discharged coolant water (thermal pollution). Industry requires pure water for many applications and utilizes a variety of purification techniques both in water supply and discharge.
Food processing

Water can be used to cook foods such as noodles.
Solutes such as salts and sugars found in water affect the physical properties of water. The boiling and freezing points of water are affected by solutes, as well as air pressure, which is in turn affected by altitude. Water boils at lower temperatures with the lower air pressure which occurs at higher elevations. One mole of sucrose (sugar) per kilogram of water raises the boiling point of water by 0.51 °C, and one mole of salt per kg raises the boiling point by 1.02 °C; similarly, increasing the number of dissolved particles lowers water’s freezing point. Solutes in water also affect water activity which affects many chemical reactions and the growth of microbes in food. Water activity can be described as a ratio of the vapor pressure of water in a solution to the vapor pressure of pure water. Solutes in water lower water activity. This is important to know because most bacterial growth ceases at low levels of water activity. Not only does microbial growth affect the safety of food but also the preservation and shelf life of food.
Water hardness is also a critical factor in food processing. It can dramatically affect the quality of a product as well as playing a role in sanitation. Water hardness is classified based on the amounts of removable calcium carbonate salt it contains per gallon. Water hardness is measured in grains; 0.064 g calcium carbonate is equivalent to one grain of hardness. Water is classified as soft if it contains 1 to 4 grains, medium if it contains 5 to 10 grains and hard if it contains 11 to 20 grains. The hardness of water may be altered or treated by using a chemical ion exchange system. The hardness of water also affects its pH balance which plays a critical role in food processing. For example, hard water prevents successful production of clear beverages. Water hardness also affects sanitation; with increasing hardness, there is a loss of effectiveness for its use as a sanitizer.
Boiling, steaming, and simmering are popular cooking methods that often require immersing food in water or its gaseous state, steam. Water is also used for dishwashing.
Water law, water politics and water crisis

An estimate of the share of people in developing countries with access to drinking water 1970–2000
1.6 billion people have gained access to a safe water source since 1990. The proportion of people in developing countries with access to safe water is calculated to have improved from 30% in 1970 to 71% in 1990, 79% in 2000 and 84% in 2004. This trend is projected to continue. To halve, by 2015, the proportion of people without sustainable access to safe drinking water is one of the Millennium Development Goals. This goal is projected to be reached.
A 2006 United Nations report stated that “there is enough water for everyone”, but that access to it is hampered by mismanagement and corruption. In addition, global initiatives to improve the efficiency of aid delivery, such as the Paris Declaration on Aid Effectiveness, have not been taken up by water sector donors as effectively as they have in education and health, potentially leaving multiple donors working on overlapping projects and recipient governments without empowerment to act.
The UN World Water Development Report (WWDR, 2003) from the World Water Assessment Program indicates that, in the next 20 years, the quantity of water available to everyone is predicted to decrease by 30%. 40% of the world’s inhabitants currently have insufficient fresh water for minimal hygiene. More than 2.2 million people died in 2000 from waterborne diseases (related to the consumption of contaminated water) or drought. In 2004, the UK charity WaterAid reported that a child dies every 15 seconds from easily preventable water-related diseases; often this means lack of sewage disposal; see toilet.
Organizations concerned with water protection include International Water Association (IWA), WaterAid, Water 1st, American Water Resources Association. Water related conventions are United Nations Convention to Combat Desertification (UNCCD), International Convention for the Prevention of Pollution from Ships, United Nations Convention on the Law of the Sea and Ramsar Convention. World Day for Water takes place on 22 March and World Ocean Day on 8 June.
Water used in the production of a good or service is virtual water.
Water in culture
Religion
Water is considered a purifier in most religions. Major faiths that incorporate ritual washing (ablution) include Christianity, Hinduism, Rastafari movement, Islam, Shinto, Taoism, and Judaism. Immersion (or aspersion or affusion) of a person in water is a central sacrament of Christianity (where it is called baptism); it is also a part of the practice of other religions, including Judaism (mikvah) and Sikhism (Amrit Sanskar). In addition, a ritual bath in pure water is performed for the dead in many religions including Judaism and Islam. In Islam, the five daily prayers can be done in most cases (see Tayammum) after completing washing certain parts of the body using clean water (wudu). In Shinto, water is used in almost all rituals to cleanse a person or an area (e.g., in the ritual of misogi). Water is mentioned in the Bible 442 times in the New International Version and 363 times in the King James Version: 2 Peter 3:5(b) states, “The earth was formed out of water and by water” (NIV). In the Qur’an it is stated that “Living things are made of water” and it is often used to described Paradise.Philosophy
The Ancient Greek philosopher Empedocles held that water is one of the four classical elements along with fire, earth and air, and was regarded as the ylem, or basic substance of the universe. Water was considered cold and moist. In the theory of the four bodily humors, water was associated with phlegm. The classical element of Water was also one of the five elements in traditional Chinese philosophy, along with earth, fire, wood, and metal.Water is also taken as a role model in some parts of traditional and popular Asian philosophy. James Legge’s 1891 translation of the Dao De Jing states “The highest excellence is like (that of) water. The excellence of water appears in its benefiting all things, and in its occupying, without striving (to the contrary), the low place which all men dislike. Hence (its way) is near to (that of) the Tao” and “There is nothing in the world more soft and weak than water, and yet for attacking things that are firm and strong there is nothing that can take precedence of it—for there is nothing (so effectual) for which it can be changed.”
Literature
Water is used in literature as a symbol of purification. Examples include the critical importance of a river in As I Lay Dying by William Faulkner and the drowning of Ophelia in Hamlet.Sherlock Holmes held that “From a drop of water, a logician could infer the possibility of an Atlantic or a Niagara without having seen or heard of one or the other.”
Rain

A rain shaft at the base of a thunderstorm
Moisture moving along three-dimensional zones of temperature and moisture contrasts known as weather fronts is the major method of rain production. If enough moisture and upward motion is present, precipitation falls from convective clouds (those with strong upward vertical motion) such as cumulonimbus (thunderstorms) which can organize into narrow rainbands. In mountainous areas, heavy precipitation is possible where upslope flow is maximized within windward sides of the terrain at elevation which forces moist air to condense and fall out as rainfall along the sides of mountains. On the leeward side of mountains, desert climates can exist due to the dry air caused by downslope flow which causes heating and drying of the air mass. The movement of the monsoon trough, or intertropical convergence zone, brings rainy seasons to savannah climes. Rain is the primary source of freshwater for most areas of the world, providing suitable conditions for diverse ecosystems, as well as water for hydroelectric power plants and crop irrigation. Rainfall is measured through the use of rain gauges. Rainfall amounts are estimated actively by weather radar and passively by weather satellites.
The urban heat island effect leads to increased rainfall, both in amounts and intensity, downwind of cities. Global warming is also causing changes in the precipitation pattern globally, including wetter conditions across eastern North America and drier conditions in the tropics. Precipitation is a major component of the water cycle, and is responsible for depositing most of the fresh water on the planet. The globally-averaged annual precipitation is 990 millimetres (39 in). Climate classification systems such as the Köppen climate classification system use average annual rainfall to help differentiate between differing climate regimes. Australia is the Earth’s driest continent. Rain is also known or suspected on other worlds, composed of methane, iron, neon, and sulfuric acid rather than water.
Fog
Fog is a cloud that is in contact with the ground. A cloud may be considered partly fog; for example, the part of a cloud that is suspended in the air above the ground is not considered fog, whereas the part of the cloud that comes in contact with higher ground is considered fog.Fog is distinguished from mist only by its density, as expressed in the resulting decrease in visibility: Fog reduces visibility to less than 1 km, whereas mist reduces visibility to no less than 1 km but less than 2 km. For aviation purposes in the UK, a visibility of less than 2 km but greater than 999 m is considered to be mist if the relative humidity is 95% or greater – below 95% haze is reported.
The foggiest place in the world is the Grand Banks off the island of Newfoundland, the meeting place of the cold Labrador Current from the north and the much warmer Gulf Stream from the south. Some of the foggiest land areas in the world include Argentina, Newfoundland, Labrador and Point Reyes, California, each with over 200 foggy days per year. Even in generally warmer southern Europe, thick fog and localized fog is often found in lowlands and valleys, such as the lower part of the Po Valley and the Arno and Tiber valleys, as well as on the Swiss plateau, especially in the Seeland area, in late autumn and winter. Other notably foggy areas include coastal Chile (in the south), coastal Namibia, and the Severnaya Zemlya islands.
Mist
Mist is a phenomenon of small droplets suspended in air. It can occur as part of natural weather or volcanic activity, and is common in cold air above warmer water, in exhaled air in the cold, and in a steam room of a sauna. It can also be created artificially with aerosol canisters if the humidity conditions are right.The only difference between mist and fog is visibility . This phenomenon is called fog if the visibility is one kilometre (1,100 yards) or less (in the UK for driving purposes the definition of fog is visibility less than 200 metres, for pilots the distance is 1 kilometre). Otherwise it is known as mist. Seen from a distance, mist is bluish, while haze is more brownish.
Religious connotations are associated with mist in some cultures; it is used as a metaphor in 2 Peter 2:17.
Mist makes a beam of light visible from the side via refraction and reflection of the suspended water droplets.
Scotch Mist refers to a light, steady drizzle, the name being typical of the Scottish penchant for understatement (and of Scottish weather).
Mist usually occurs near the shores, and is often associated with fog. Mist can also be as high as mountain tops when extreme temperatures are low.




